Showing posts with label endorphins. Show all posts
Showing posts with label endorphins. Show all posts
Monday, October 12, 2015
Cannabis Receptors and the Runner’s High
[First published August 4 2010]
Maybe it isn't endorphins after all.
What do long-distance running and
marijuana smoking have in common? Quite possibly, more than you’d think.
A growing body of research suggests that the runner’s high and the
cannabis high are more similar than previously imagined.
The nature of the runner’s high is
inconsistent and ephemeral, involving several key neurotransmitters and
hormones, and therefore difficult to measure. Much of the evidence comes
in the form of animal models. Endocannabinoids—the body’s internal
cannabis—“seem to contribute to the motivational aspects of voluntary running in rodents.” Knockout mice lacking the cannabinioid CB1 receptor, it turns out, spend less time wheel running than normal mice.
A Canadian neuroscientist who blogs as NeuroKuz
suggests that “a reduction in CB1 levels could lead to less binding of
endocannabinoids to receptors in brain circuits that drive motivation to
exercise.” NeuroKuz speculates on why this might be the case. Physical
activity and obtaining rewards are clearly linked. The fittest and
fleetest obtain the most food. “A possible explanation for the runner’s
high, or ‘second wind,’ a feeling of intense euphoria associated with
going on a long run, is that our brains are stuck thinking that lots of
exercise should be accompanied by a reward.”
In 2004, the British Journal of Sports Medicine
ran a research review, “Endocannabinoids and exercise,” which seriously
disputed the “endorphin hypothesis” assumed to be behind the runner’s
high. To begin with, other studies have shown that exercise activates the endocannabinoid system.
“In recent years,” according to the
authors, “several prominent endorphin researchers—for example, Dr Huda
Akil and Dr. Solomon Snyder—have publicly criticised the hypothesis as
being ‘overly simplistic,’ being ‘poorly supported by scientific
evidence’, and a ‘myth perpetrated by pop culture.’” The primary
problem is that the opioid system is responsible for respiratory
depression, pinpoint pupils, and other effects distinctly unhelpful to
runners.
The investigators wired up college
students and put them to work in the gym, and found that “exercise of
moderate intensity dramatically increased concentrations of anandamide
in blood plasma.” The researchers break the runner’s high into four
major components. Exercise, they say, “suppresses pain, induces
sedation, reduces stress, and elevates mood.” Some of the parallels
with the cannabis high are not hard to tease out: “Analgesia, sedation
(post-exercise calm or glow), a reduction in anxiety, euphoria, and
difficulties in estimating the passage of time.”
There are cannabinoid receptors in
muscles, skin and the lungs. Intriguingly, the authors suggest that
unlike “other rhythmic endurance activities such as swimming, running is
a weight bearing sport in which the feet must absorb the ‘pounding of
the pavement.’” Swimming, the authors speculate, “may not stimulate
endocannabinoid release to as great an extent as running.” Moreover,
“cannabinoids produce neither the respiratory depression, meiosis, or
strong inhibition of gastrointestinal motility associated with opiates
and opioids. This is because there are few CB1 receptors in the
brainstem and, apparently, the large intestine.”
A big question remains: What about running and the “motor inhibition” characteristic of high-dose cannabis?
(An inhibition that may make cannabis useful in the treatment of
movement disorders like tremors or tics.) Running a marathon is not the
first thing on the minds of most people after getting high on
marijuana. The paper maintains, however, that at low doses,
“cannabinoids tend to produce hyperactivity,” at least in animal models.
The CB1 knockout mice were abnormally inactive, due to the effect of
cannabinoids on the basal ganglia. Practiced, automatic motor skills
like running are controlled in part by the basal ganglia. The authors
predict that “low level skills such as running, which are controlled to
a higher degree by the basal ganglia than high level skills, such as
basketball, hockey, or tennis, may more readily activate the
endocannabinoid system.”
The authors offer other intriguing
bits of evidence. Anandamide, one of the brain’s own cannabinoids, “acts
as a vasodilator and products hypotension, and may thus facilitate
blood flow during exercise.” In addition, “endocannabinoids and
exogenous cannabinoids act as bronchodilators” and could conceivably
facilitate breathing during steady exercise. The authors conclude:
“Compared with the opioid analgesics, the analgesia produced by the
endocannabinoid system is more consistent with exercise induced
analgesia.”
Photo Credit: http://www.madetorun.com/
Thursday, November 29, 2007
Naloxone and “Receptorology”
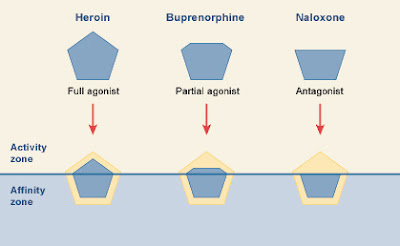
The power of the opiates revealed
The breakthrough that laid the groundwork for the first truly scientific understanding of addictive drugs took place in 1972, when researchers discovered the existence of specific receptor sites in the brain for the opium molecule.
At roughly the same time, emergency room doctors were baffled to discover that timely injections of a drug called naloxone completely reversed the effects of heroin intoxication. Minutes after an injection of naloxone, heroin addicts were awake, fully recovered, and instantly into the rigors of heroin withdrawal. Naloxone, and a similar drug called naltrexone, rescued O.D. victims from respiratory failure. Like a magic bullet, naloxone--trade name Narcan-- blocked the effects of heroin.
At Johns Hopkins University School of Medicine in Baltimore, Dr. Solomon Snyder and a young doctoral candidate named Candace Pert devised a method for testing this theory. By making molecules of naloxone radioactive, and following the course of the molecules with the aid of a radiation counter, Snyder and Pert were able to show that naloxone attached itself very specifically to certain neurons in certain parts of the brain. If naloxone molecules were capable of locking into specific sites, then presumably these were the same sites in the brain where the opiates did their work.
The sites in question were mean for naturally occurring painkillers called endorphins. The only reason opium worked so dramatically to relieve pain was because a part of the opium molecule was similar in shape to the naturally occurring endorphins. Heroin “fooled” the receptors designed for the shape of an endorphin molecule. Not only that, but heroin and the other opiates stimulated these receptors just as effectively as the natural endorphins did.
The stunning power of the opiates had been revealed as an architectural quirk of nature.
Naloxone was a heroin antagonist—it blocked the effect of the drug at specific sites on nerve cells in the brain. (If the drug fits the receptor and elicits a response, it is called an agonist. If it simply blocks the receptor site without stimulating a response, it is an antagonist.)
The naloxone molecule also bore an uncanny resemblance to the shape of natural endorphin molecules, and when doctors gave an O.D. victim a shot of naloxone, the naloxone molecules knocked the opium molecules right off their receptors. Then they bound themselves to the endorphin sites even more tightly than the heroin molecules did. Naloxone was capable of snapping onto the receptor sites without triggering the release of endorphin.
The brain scans developed for studying this chemical activity were produced by introducing radioactive atoms into naloxone. Wherever naloxone stuck to a receptor site in the brain of a rat, the “hot” connection lit up on special film. These maps of receptor geography in the brain led Dr. Pert and her colleagues to christen the new science “receptorology.” Likening these snapshots to “tiny sparkling grains in a sea of colorfully stained brain tissue,” Pert was helping to invent a new field of study.
“Receptorology” came to be known as neuroscience, or neuropharmacology, and operated under a deceptively simple premise: If it is a drug, and if it has an effect on the brain, then it must have a brain receptor site to which it binds. Find its site of action, and you find out what it is, what it does, and where it does it.
Labels:
addiction science,
endorphins,
heroin addiction,
naloxone,
narcan,
receptors
Subscribe to:
Posts (Atom)
